Production
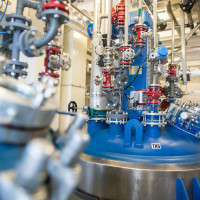
Production Facilities
At FARMAK, a.s. the production is realized in several production plants in compliance with the cGMP and ISO systems. Each of them includes several production modules used mainly for multi-purpose use (for the production of several different products). We have manufacturing facilities in stainless steel, enamel, or Hastelloy C22, which enables the production of a batch size from 100 g up to several kilograms (Kilolab) and from 5 kg to 250 kg in reactors with a capacity from 250 to 6300 litres (the standard volume of the reactors is 1600 litres).
For the API produced by us we also provide particle size adjustment (from sieving through various grinding stages to micronization), which, together with the final packaging of the products, is performed in a separate production facility.

Technology
Established Technologies
The production programme specializes mainly in the chemistry of heterocyclic compounds. For our current production line of API, we have introduced Industrial processes for many basic chemical reactions, such as:
Grignard reactions (including sulphur)
various condensations
Friedel-Crafts reactions
halogenation
oxidation
reduction (Zn)
esterification
separation of racemic mixtures
low-pressure hydrogenation
reaction in the presence of a phase catalyst
enzymatic reactions (one application yet)
The reactions are carried out at temperatures of -20 °C to +140 °C (in some reactors with thermostats at temperatures of -90 °C to +300 °C) and at a pressure of -80 to +300 kPa (with vacuum up to 5 mbar of absolute pressure in some apparatuses).
New Technologies
In addition to the established technologies, we also focus on searching for other products requiring new technologies. For their successful development, optimization, and transfer to production scale (with maximum efficiency in terms of cost and time), we have formed a strong background consisting in particular of:
a creative team of production and production technology specialists
an extensive research and development department
suitable production equipment for gradual scale-up (laboratory reactors, Kilolab, pilot plant)
the necessary facilities (a well-equipped analytical centre – NMR, GC-MS, HPLC, DSC, laser diffraction), and a team of quality assurance experts and others
mutual cooperation with a number of universities and other research centres
 Fotogalerie
Fotogalerie